Proxy systems
Soluble chemical diffusion in polar ice
When one measures the concentration of a chemical species in ancient glacial ice, it is typically assumed that that chemical species was atmospherically deposited at the same time as the surrounding snow and ice in which it is measured. Yet, this is not always the case. When soluble chemical species mix with water, new “aqueous solutions” are developed with unique melting points. This is a fact most of know from our daily lives: when roads freeze over during winter, we salt the roads to “suppress” the freezing point of water. This turns the ice to slush even when temperatures are less than 0oC (32oF). A similar thing can happen in glacial ice containing soluble chemical species. When it does, the formation of microscopic liquid pathways enables chemical ions to rapidly travel away from the snow and ice it was originally deposited. This creates an obvious problem for ice core climate scientists: if we don’t know when or where the chemical species was originally deposited, how can we ever hope to interpret its climatically?
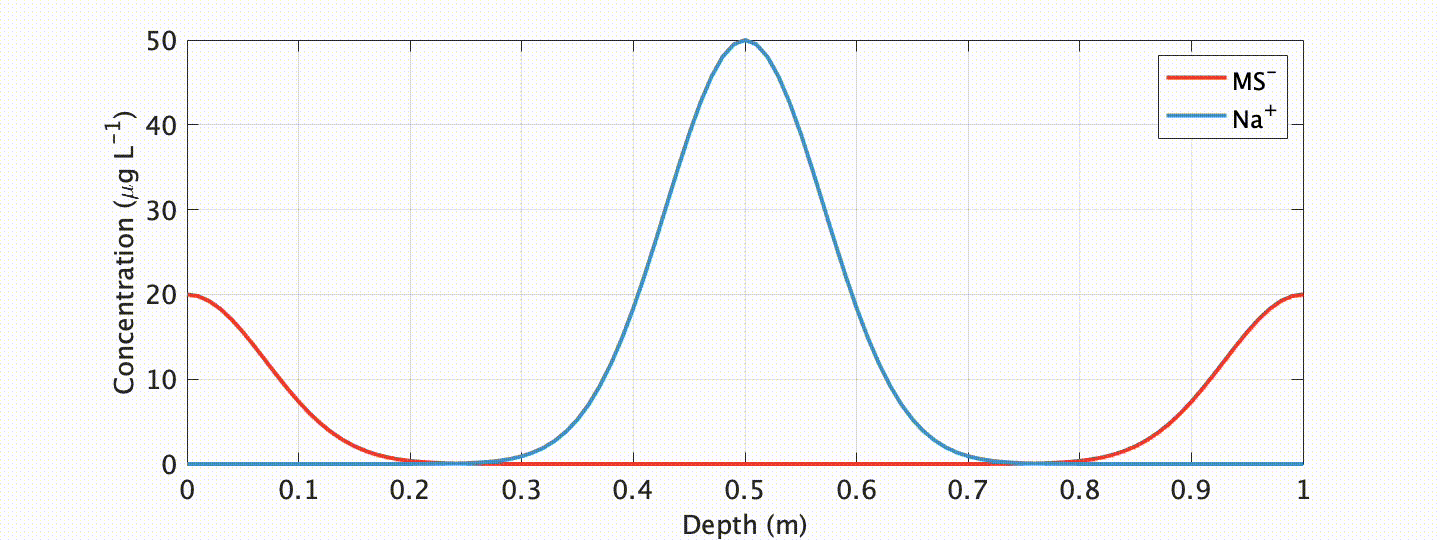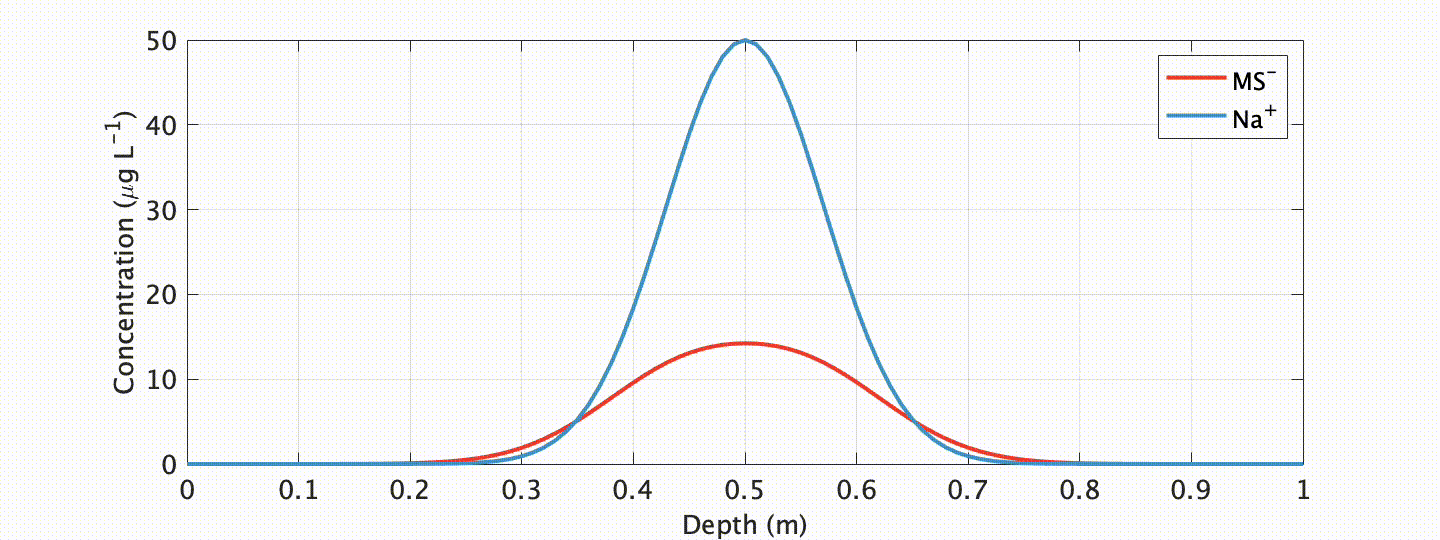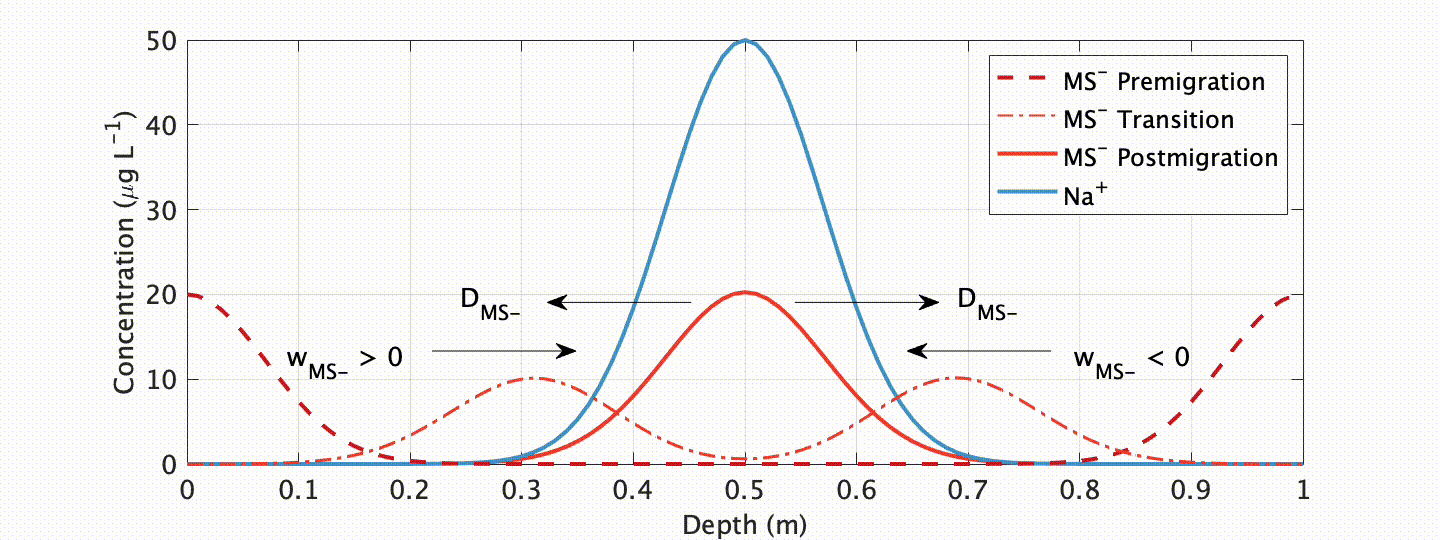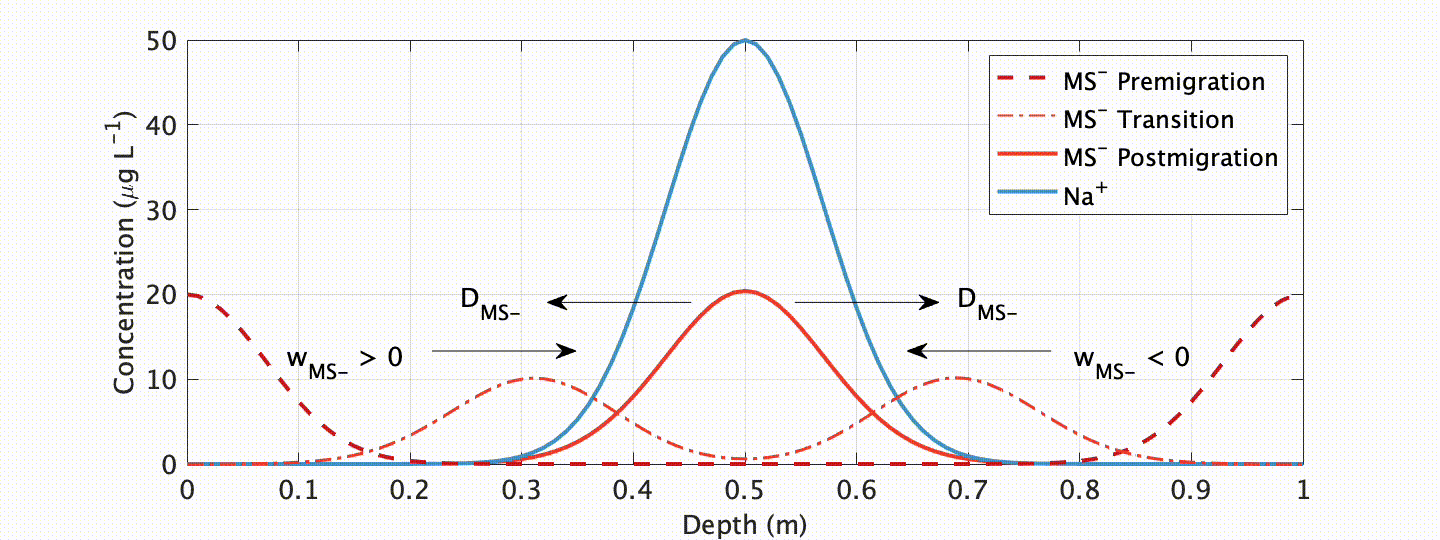
One ionic species showing especially unique behavior is methanesulfonate (MS-). MS- has long been targeted as a climate proxy in ice cores because it is near-solely sourced from ocean plankton. Due to this unique source, and the fact that plankton only bloom when adequate temperature and sunlight exists, at high-latitudes it is near-solely deposited in summer snow/ice layers. Progressively deeper below the glacier surface, however, MS- is known to migrate to winter layers.
In an exhaustive study published in The Cryosphere, myself and colleagues at the Woods Hole Oceanographic Institution explored this curious, but problematic, phenomenon. We showed that such migration is closely linked to the impurity content of the cationic species Na+ in polar ice (which often peaks in winter), and developed a physicochemical advection-diffusion model ice flow model to accurately simulate the process.
What are the applications of our model? Well, first, it allows us to assess the integrity of a given (MS-) chemical record! This is an important requirement if we want to interpret this record as an indicator of past climate. Our model shows that migration is mitigated at sites with higher snow accumulation and (or) lower impurity content. Another exciting, possible future application? With our proxy model comes the possibility of “correcting” for chemical migration by “back-diffusion” , or “forward modeling” geochemical proxies for data assimilation applications.
For more work on a novel climatic application of the MS- proxy, please see our follow-up study on reconstructing North Atlantic productivity using Greenland ice sheet MS- ice core records, here.
Arctic sea ice from sea sediment geochemical proxies
More to follow!
References
- Osman, M., Das, S. B., Marchal, O., and Evans, M. J.: Methanesulfonic acid (MSA) migration in polar ice: Data synthesis and theory, The Cryosphere, 11, 2439-2462, 2017.